Disturbances in the wiring diagram: Researchers have discovered a vulnerability in our genome that could lead to developmental problems such as extra fingers and heart disease. Accordingly, individual mutations in certain sections of DNA, i.e. enhancers, can change gene expression instructions, the researchers report in the journal Nature. As a result, faulty proteins are produced, thus disrupting physical growth.
Our genome contains precise instructions for how our bodies grow and develop. Millions of genomic switches, called enhancers, control which genes should be active at what time and in what part of the body. This in turn ensures that the right proteins are produced in the right cells at the right time throughout our lives.
If these switches are changed or faulty, developmental problems and diseases can occur. In fact, most genetic diseases result from mutations and changes in these enhancers, as previous studies have shown. But not all enhancing variants are automatically harmful, some mutations also have no effect. However, distinguishing between relevant changes and irrelevant changes has so far been like searching for a needle in a haystack for researchers.
How do genomic switches work?
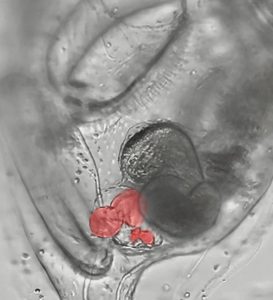
A research group led by Fabian Lim of the University of California, San Diego, has examined how reinforcers work in more detail. To do this, the researchers specifically analyzed an enhancer called ZRS, which is known to associate with extra limbs in humans and mice.
In mouse models and various in vitro experiments, biomedical scientists first investigated which gene the ZRS enhancer activates and the molecular mechanism by which this happens. The biomedical scientists then compared how this mechanism changes when there are different point mutations in the DNA section of the enhancer.
Bond density is critical
The ZRS genetic switch has been shown to activate expression of the Shh gene by binding specific proteins, so-called transcription factors – but surprisingly only very weakly. In subsequent experiments, Lim and his colleagues found the same principle with other enhancers and their transcription factors. Thanks to weak binding, enhancers can usually fine-tune which gene should be active, where, at what time, and to what extent, the researchers concluded.
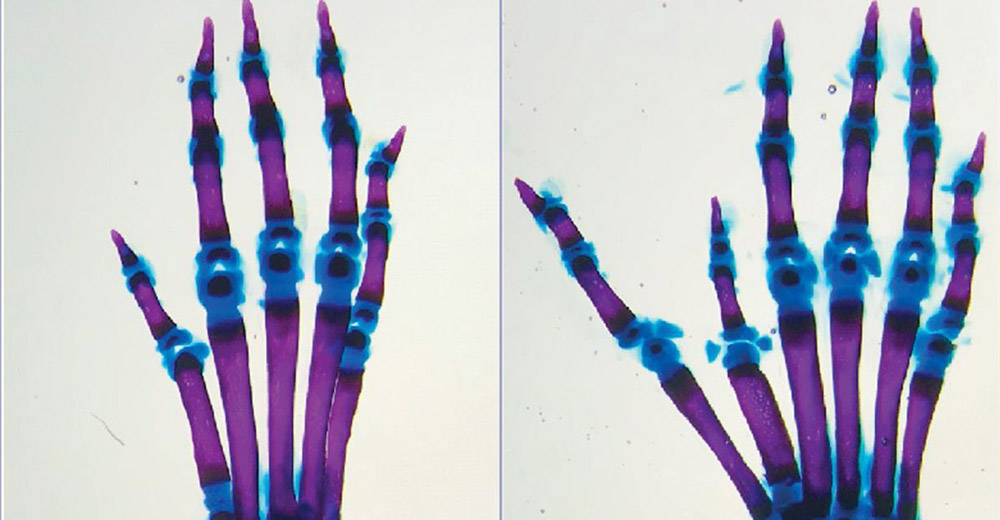
However, if a point mutation occurred within the genomic section of the ZRS enhancer, causing a base pair exchange, transcription factors were able to bind more strongly to the enhancer in some cases examined. This effect can also be transferred to other reinforcers, as subsequent experiments have shown. In all cases, fine-tuning of gene regulation was lost due to stronger binding to the transcription factor, Lim and colleagues reported. This led to more or less dramatic developmental problems – with the ZRS-Enhancer device, for example, one or two extra fingers.
Targeted search for mutated enhancers is possible
“Our study highlights a central weakness in our genome: single base pair changes that cause transcription factors to bind slightly more strongly to the enhancer can lead to developmental disorders,” says lead researcher Emma Farley from the University of California, San Diego. However, mutations in enhancers that led to weaker binding of transcription factors did not lead to developmental disorders.
“By specifically looking for DNA base pair changes in enhancers that lead to stronger binding of transcription factors, we can now find health-relevant enhancer variants much more quickly,” she adds. Researchers have developed a special test that can be used to find such mutations more quickly.
The consequences of harmful mutations are predictable
Using this test, biomedical scientists have already compared different genomes and enhancer variants. Based on the results, they can now predict in detail which enhancing mutations might lead to changes in gene expression and what consequences this might have on physical development. This applies not only to changes in the limbs and not only to humans. For example, in a second study, Lim and colleagues found that sea squirts develop a second heart when a certain mutation occurs in heart growth promoters.
Knowing how genetic switches now work also opens new opportunities for personalized medicine, according to the researchers. “Using this knowledge will allow us to better predict enhancing variants that underlie the disease,” Farley says. (Nature, 2024; doi: 10.1038/s41586-023-06922-8)
Source: University of California, San Diego
February 7, 2024 – Claudia Crabb

“Total coffee aficionado. Travel buff. Music ninja. Bacon nerd. Beeraholic.”







More Stories
Coral Seeding: Artificial Insemination Makes Coral More Heat Tolerant
Fear, Anger, and Denial: How People Respond to Climate Change – Research
LKH Graz: Using radiation to combat heart arrhythmias