Researchers discover biomarkers of brain damage in extremely premature babies at an early stage
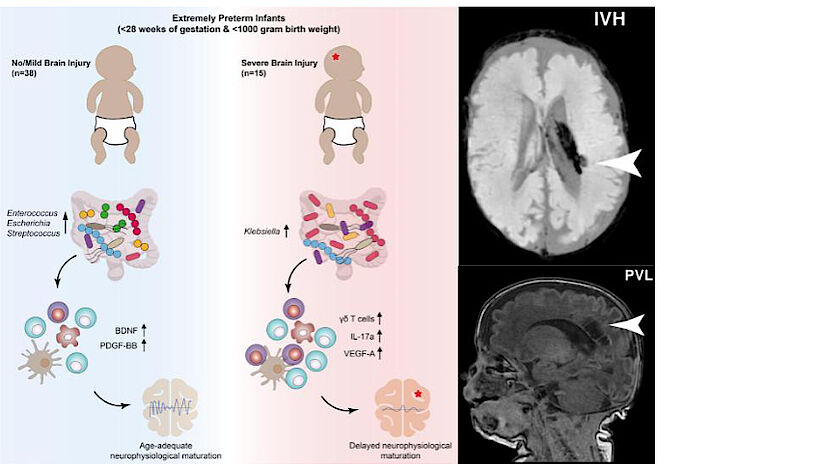
Very premature babies are at risk of developing brain damage. Researchers at the University of Vienna and the Medical University of Vienna have now found starting points for early treatment of such damage outside the brain: Bacteria in the gut of premature babies play a major role in this. The research team found that GI overgrowth with Klebsiella bacteria is associated with increased levels of certain immune cells and the development of neurological damage in premature infants. The study has now been published in the journal Cell Host & Microbe.
Complex interaction: gut axis, immune system and brain
The early development of the gut, brain, and immune system are closely related. The research talks about the gut-immune brain axis. The bacteria in the gut cooperate with the immune system, which monitors the gut microbiome and develops appropriate reactions to it. The intestines come into contact with the brain via the vagus nerve, but also via the immune system. “We investigated the role this axis plays in the brain development of very premature infants,” says the study’s lead author, David Seke. “The microorganisms of the gut microbiome – the bioaccumulation of hundreds of species of bacteria, fungi, viruses and other microbes in the gut – are balanced in healthy people. Especially in premature babies, whose immune system and microbiome are not fully developed, here but it is likely to be mutations, which can also have negative effects on the brain,” says the microbiologist and immunologist.
Patterns in the microbiome indicate brain damage
Adds David Berry, microbiologist and head of the research group at the Center for Microbiology and Ecosystem Sciences at the University of Vienna, and head of the Shared Microbiome Facility – a scientific association between the University of Vienna and the Medical University of Vienna. “It is now important that such patterns appear more often before changes in the brain. This opens a critical time window during which brain damage in very premature infants can be avoided or prevented from getting worse.”
A comprehensive study of the development of extremely preterm infants
The starting point for developing appropriate treatments are the biomarkers that the multidisciplinary team has been able to identify. Lukas Wisgrill, MD, specialist in neonatology at the Department of Neonatalogy, Pediatric Intensive Care Medicine and Neuropediatrics at the Children’s University Hospital of the Medical University of Vienna explains. “We’ve been able to track these patterns because, for the first time, we’ve done detailed research on how the gut microbiome, the immune system and the brain develop and how they interact with a very specific group of newborns,” he adds. The study followed a total of 60 extremely premature babies born before the 28th week of pregnancy and weighing less than one kilogram for several weeks, sometimes months. Using the latest methods, the researchers analyzed the microbiome, immune cells, brain wave measurements (such as aEEG) and MRI images of the children’s brains.
The research continues in two studies
More surveys will be linked here on two levels: the study, which was conducted as a joint collaborative project between universities under the joint leadership of Angelica Berger (Comprehensive Center for Pediatrics, Medical University of Vienna) and David Berry (University of Vienna), is the starting point for a research project examining the microbiome and its importance for the neurological development of preterm infants more comprehensively. In addition, the researchers would like to continue to accompany the children in the initial study. “How children’s motor and cognitive abilities develop can only be seen over several years,” explains Angelica Berger. “We would like to better understand how this very early development of the gut-immune-system-brain axis affects the long-term.” The most important collaborative partners in the project are already in place: “The parents of the children supported us in the study with great interest and openness,” says David Seke. “Ultimately, that was the only reason we were able to gain these important insights. We are very grateful for that.”
Deployment in Cell Host and Microbe:
“Evolution of the aberrant gut microbiota-immunity-brain axis in preterm neonates with brain damage.” David Seke, Margarita Meyer, Bella Hausmann, Petra Bjevac, Vito Giordano, Katharina Gueral, Lucas Unterazinger, Catherine Kleber, Maes Schrehoff, Kim DeBaby, Tom Van de Weele, Andreas Spitler, Gregor Kasprian, Benedict Warth, Angelica Berger, David Berry and Lucas Whisgril. Host Cell and Microbe (2021), DOI: 10.1016/j.chom.2021.08.04

“Total coffee aficionado. Travel buff. Music ninja. Bacon nerd. Beeraholic.”







More Stories
Coral Seeding: Artificial Insemination Makes Coral More Heat Tolerant
Fear, Anger, and Denial: How People Respond to Climate Change – Research
LKH Graz: Using radiation to combat heart arrhythmias