Like the search team around Roland Wester This is the slowest interaction ever observed with charged particles, he and his team from the University of Innsbruck’s Institute of Ion Physics and Applied Physics report in the journal Nature.
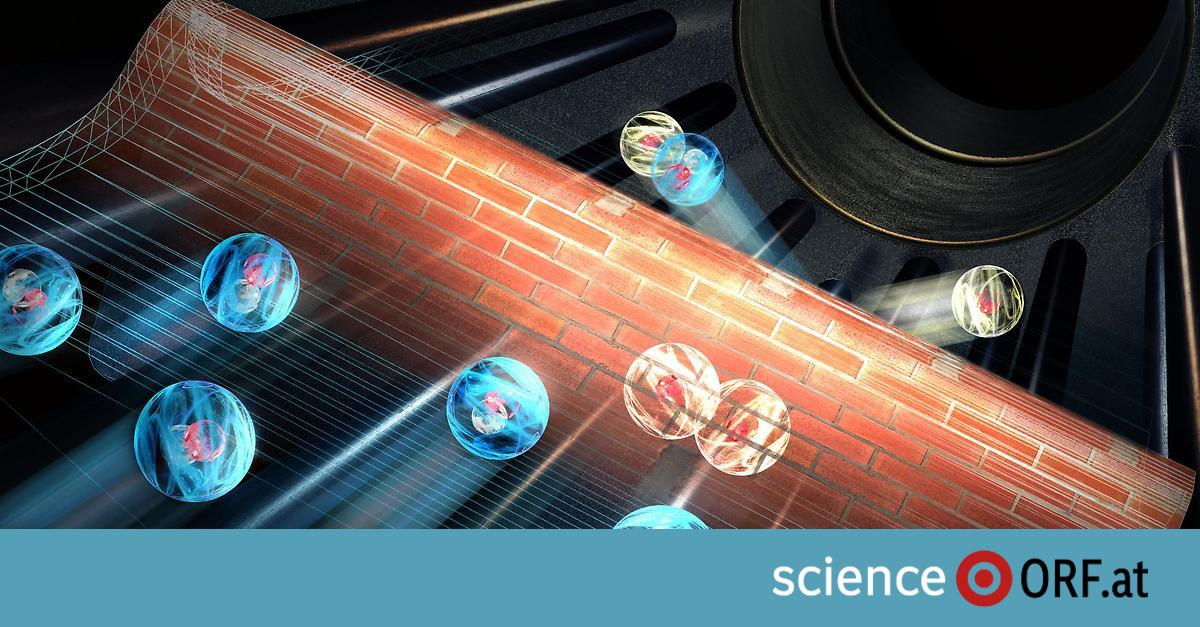
It has been known for nearly 100 years that a particle can overcome a barrier even if it does not have the energy to do so. The background to this “tunnel effect” is that in quantum mechanics, the exact position and velocity of a particle cannot be determined at the same time. One can only make probabilistic data. Therefore, an atom, for example, can also be behind an energy barrier with a certain probability.
Technologically, this quantum effect is already being used in several ways, for example in scanning tunneling microscopy and in flash memory. Alpha decay of the atomic nucleus can also be explained by it.
The tunnel effect is also in chemical reactions
The tunnel effect also plays a role in chemical reactions, for example when chemical reaction barriers are overcome as a result. However, it is very difficult to predict tunnel reactions. Since it is difficult to quantum mechanically accurately describe a chemical reaction with more than three particles, it is almost impossible with more than four particles.
Wester and his team investigated Stady One of the fundamental molecular interactions – namely between charged atomic hydrogen and molecular hydrogen. This simple interaction can be fully described in quantum mechanics. To do this, they used deuterium, that is, an isotope of hydrogen with an extra neutron in the nucleus. This isotope is negatively charged, that is, to form an ion, it is placed in an ion trap and cooled to about minus 263 degrees Celsius. The trap is then filled with hydrogen gas, which consists of molecules with two hydrogen atoms each.
Despite the low temperatures, there are many collisions, but deuterium ions lack the energy to interact with hydrogen molecules in the traditional way. However, in very rare cases, this is caused by the tunneling effect: “Quantum mechanics allows particles to break through the energy barrier and a reaction occurs,” first author Robert Wilde of the Wester team explained in a broadcast. This reaction produces negatively charged hydrogen ions and deuterium hydrogen molecules.
The slowest interaction with ions ever observed
“We let the experiment run for about 15 minutes — that’s much longer than is technically possible in most laboratories around the world for these ions,” says Wester. The researchers then determined the number of hydrogen ions formed, from which they could infer how often the reaction occurred. Over the long observation period, less than one percent of the particles reacted chemically—making it the slowest-reacting ion on record.
It’s relatively slow, however, because it also depends on how much hydrogen is presented to the deuterium ion as a reaction partner, according to Wester. So it would be more correct to talk about the probability of a collision reaction out of 100 billion – a tunneling reaction rarely happens. This value is consistent with one calculated by theoretical physicists in 2018. According to the researchers, this is the first time that an accurate theoretical model of the tunnel effect in a chemical reaction has been confirmed.
From this first measurement of the tunnel reaction, which is also well understood in theory, scientists hope to gain a better understanding of other chemical reactions behind which the tunnel effect is suspected.

“Total coffee aficionado. Travel buff. Music ninja. Bacon nerd. Beeraholic.”







More Stories
Coral Seeding: Artificial Insemination Makes Coral More Heat Tolerant
Fear, Anger, and Denial: How People Respond to Climate Change – Research
LKH Graz: Using radiation to combat heart arrhythmias